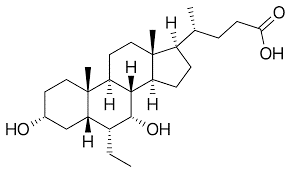
Obeticholic acid from Bacto Chem
Bacto Chem’s Obeticholic acid is manufactured domestically in India Using Indian bovine-source Bile, which is used to manufacture cholic acid, the key starting material in the manufacturing process of UDCA. Our patented manufacturing process gives us an edge over our competitors owing to our process’ superiority in terms of yield and quality.
Indian cattle are often recognised and believed to be better for human consumption because:
Absence of Bovine Spongiform Encephalopathy (BSE), or Mad Cow Disease
• BSE is caused in cattle when the animals are fed with meat or bone meal. Protein cells become twisted into prions. Prions are NOT DESTROYED by fire, freezing, disinfectants, sterilization procedures or radiation.
• Indian cattle are grass fed, this factor removes the possibility of these cattle encountering BSE,
• Cholic Acid prepared from Indian cattle is free of BSE/TSE.
Greater bioavailability:
• Bovine sourced proteins and APIs show greater affinity of binding with human cells to show greater affinity of binding, showing greater efficacy.
All Bacto Chem Products are manufactured in compliance with the World Health Organization (WHO) Current Good Manufacturing Practices (cGMP) under strict conditions in state-of-the-art facilities. This results in products that are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification. Each of our products is manufactured in a dedicated block with no cross-contamination.
Our strengths lie in our highly trained and experienced QC department which ensures all the raw materials go through stringent testing prior to their utilisation, ensuring that only raw materials of the highest quality are used only after approval from QC. We ensure that all our equipment and manufacturing process are thoroughly validated and updated. We invest heavily on training our production and QC teams to understand and monitor every stage of the production cycle to ensure safety and highest quality product. Maintaining cleanliness is our responsibility, and we always focus on ensuring proper measures are taken to follow our validates cleaning protocols to avoid batch to batch contamination.
Additionally, Bacto Chem is compliant with the requirements of OHSAS 18001:2007 in occupational health and safety management systems. Bacto Chem is also ISO 14001:2015 certified which recognizes us as an organization seeking to manage its environmental responsibilities in a systematic manner that contributes to the environmental pillar of sustainability and has the necessary Halal certifications as well.