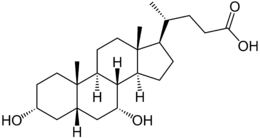
Chenodeoxycholic Acid
Chenodeoxycholic Acid is a bile acid naturally found in the body. This product is a derivative of the Cholic Acid API and is the first derivative from Cholic Acid, is the first in a large list of cholic acid derivatives. Chenodeoxycholic acid (or Chenodiol) is an epimer of ursodeoxycholic acid.
Chenodeoxycholic acid is a bile acid naturally found in the body. Chenodeoxycholic acid is a bile acid mainly found in the bile of vertebrates and mammals. Initially isolated from the bile of domestic geese, chenodeoxycholic acid is a type of carboxylic acid typically used to treat gallstones.
Along with cholic acid, chenodeoxycholic acid sodium salt is one of two primary human bile acids. Chenodeoxycholic acid contains two hydroxyl groups and is altered by adding another hydroxyl group to create cholic acid.
Chenodeoxycholic acid is produced as a white crystalline substance. It is not soluble in water, however can be dissolved in acetic acid and alcohol, and has a 165–167 °C melting point.
It works by dissolving the cholesterol that makes gallstones and inhibiting production of cholesterol in the liver and absorption in the intestines, which helps to decrease the formation of gallstones. It can also reduce the amount of other bile acids that can be harmful to liver cells when levels are elevated. It works by dissolving the cholesterol that makes gallstones and inhibiting production of cholesterol in the liver and absorption in the intestines, which helps to decrease the formation of gallstones.
Chenodeoxycholic acid is indicated for the treatment of inborn errors of primary bile acid synthesis due to sterol 27 hydroxylase deficiency (presenting as cerebrotendinous xanthomatosis (CTX)) in infants, children and adolescents aged 1 month to 18 years and adults. Chenodeoxycholic Acid is indicated for patients with radiolucent stones in well-opacifying gallbladders, in whom selective surgery would be undertaken except for the presence of increased surgical risk due to systemic disease or age. Chenodeoxycholic Acid will not dissolve calcified (radiopaque) or radiolucent bile pigment stones.